Nuheara delivers strong product growth as US market potential expands
Click here to download a PDF copy.
Key highlights – Q2 FY22
• Customer product receipts up 61% to $1.7 million (Q2 FY21: $1.0 million) underpinned by strong international sales, being 75% of total sales in Q2 FY22.
• Invoiced product revenue up 37% to $1.8 million (Q2 FY21: $1.3 million) reflecting resurgent growth in Traditional Retail (TR) sales.
• Driven by the TR sales rebound in the US, TR sales represented 43% ($0.8 million) of total sales in Q2 FY22 up from 22% in Q2 FY21.
• 12% uplift in Average Selling Price (ASP) of IQbuds² MAX implemented, increasing ASP to $453 from $405 (FY21) transitioning into higher value medical device territory and away from price sensitive earbud market.
• Nuheara’s addressable market in the US opened up to 30 million potential customers following US FDA issuing its landmark ruling to establish a new category of over-the-counter (OTC) hearing aids.
• Best Buy launches Nuheara products in 241 retail stores in US, targeting in store purchases with new Hearing Solutions category.
• Global supply agreement with Sonova AG for Nuheara products to be made available to all Sonova clinics and affiliates (total 3,200 potential Points of Sales).
• Commenced medical device clinical trial of hearing aid, providing pathway to FDA 510(k) submission.
• Built strong inventory levels, with more than $6 million worth of stock on hand (based on current ASPs) in 5 global warehouses at end of quarter.
• $5.7 million capital raised to fund US growth opportunities (including $1.07 million from Share Purchase Plan (SPP) announced on 23 December 2021 that closed on 17 January 2022).
• R&D tax rebate of $1.7 million received.
• Strong balance sheet with $6.3 million cash at 31 December 2021 (excludes funds raised in SPP).
Nuhear a Co-founder & CEO Justin Miller said:
“The second quarter was an incredibly busy and successful period for Nuheara, laying the foundations for an exciting global growth path with a fo cus on the US market.
The strength of our product offering and underlying business was evident in the strong growth in cash receipts and invoiced product sales over the second quarter of FY22.
With the retail sector reopening in the second quarter, we saw a resurgence in retail sales, highlighting the strength of Nuheara’s multi-distribution channel business model.
Building on our presence in Best Buy’s online store, Nuheara products were launched in 241 Best Buy retail stores in the US through their new Hearing Solutions category. Our retail footprint further grew with the global supply agreement put in place with Sonova.
Nuheara’s international hearing retail experience places us in a leadership position as we expand our horizons to the regulated hearing aid market in the US. With clinical trials now concluded and new US regulations in place for the second half of 2022, we look forward to providing hearable and prospective hearing aid solutions to what is expected to be a large new OTC market opportunity.”

Perth, 31 January 2022: Nuheara Limited (ASX: NUH), transforming the way people hear by creating smart and affordable hearing solutions, is pleased to present this quarterly activities report alongside its Appendix 4C for the quarter ended 31 December 2021 (Q2 FY22).
SALES AND REVENUE
Nuheara continued to deliver strong sales growth in Q2 FY22, with total invoiced product sales of $1.8 million, a 52% increase over the previous quarter (Q1 FY22) and a 37% increase over the same quarter last year (Q2 FY21).
The underlying growth of the business is better reflected in the 61% increase in Q2 customer product receipts, from $1.0 million in Q2 FY21 to $1.7 million in Q2 FY22.
In comparison, total cash receipts of $4.8 million were received in Q2 FY21. This comprised $3.8 million in OEM/HP services receipts plus the $1.0 million customer product receipts.
Price increases for IQbuds² MAX were implemented last quarter in most regions around the world. This translated to a 12% increase in the Average Selling Price (ASP) from $405 per unit in FY21 to $453 per unit this year to date. These increases have allowed the business to maintain its gross margins despite increasing global price pressures on supply chain and logistics. This is also a positive sign that Nuheara can compete in the hearing aid space with market leading products at significantly higher price points.
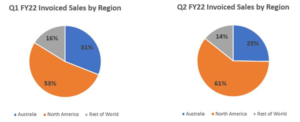
Sales by Region
International sales over the Northern hemisphere summer gained momentum with 75% of all sales in Q2 FY22 being offshore (Q1 FY22: 69%). North America accounted for 56% of sales for the quarter (Q1 FY22: 50%). Domestic product sales declined slightly over the quarter with 25% of all sales in Q2 FY22 originating in Australia, down from 30% in Q2 FY21 and 31% in Q1 FY22.
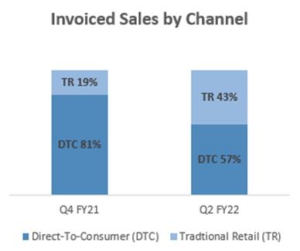
Sales by Channel
Nuheara’s hybrid channel strategy continued to deliver on its strategic intent to reach the consumer through multiple sales channels. Global sales by channel changed dramatically over the course of the half year, in part fuelled by the re-opening of traditional retail stores in the US as they returned from COVID-19 related closures, while Australian stores were still closed due to lockdowns. OEM did not contribute during the quarter.
An Omicron surge in COVID cases provided further variability to the sales by channel, as traditional retail store hours in the US were limited throughout December. This provided a short-term increase in direct-to-consumer (DTC) sales and again validated the necessity and value of a diversified sales channel.
NUHEARA’S ADDRESSABLE MARKET IN THE US OPENED UP TO 30 MILLION POTENTIAL CUSTOMERS FOLLOWING US FDA ISSUING A LANDMARK PROPOSAL FOR OTC HEARING AIDS
On 21 October 2021, Nuheara welcomed the US Food and Drug Administration (FDA) issuing its landmark proposal intended to improve access to, and reduce the cost of, hearing aid technology for millions of Americans1. The US FDA has proposed a rule to establish a new category of over-the-counter (OTC) hearing aids.
The proposed rule opens up a very substantial market opportunity for Nuheara. Approximately 37.5 million American adults aged 18 and over report some trouble hearing, yet only about one-fifth of people who could benefit from a hearing aid use one2. This leaves 30 million Americans that don’t use hearing aids given cost, access, social stigma related to hearing loss, perceived value of the devices or certain US State and Federal regulations.
The proposed rule is now open for public comment for a period of 90 days, after which the FDA will review the comments which could take up to 120 days. Once the review is complete and the final rule published, an allowance of a further 60 days is made before the rule is in effect.
This new OTC ruling, along with Nuheara’s experience, technology, and the clinical trial (see below) places the Company in the prime position to deliver more accessible and affordable hearing devices to the 30 million Americans that suffer from some form of hearing loss and currently do not use hearing aids. The Company is uniquely placed to rapidly grow once the new rule is finalised, and importantly improve US consumers’ lives with Nuheara’s hearing solutions.
NUHEARA LAUNCHES IN 241 BEST BUY STORES IN THE US
In late November 2021, Nuheara announced that its suite of products was now available within dedicated “Hearing Solutions” displays located in 241 select Best Buy stores across the United States of America.
The expansion of Nuheara’s relationship with Best Buy was significant on several fronts:
1) Nuheara’s products moved from online only sales at Best Buy to online PLUS in store across 241 Best Buy retail stores across the US
2) The adoption of a “hearing solutions” retail category in store by Best Buy reflects the growing demand for hearing products by consumers
3) The combination of the in-store presence with the new “hearing solutions” retail category provides a clear pathway for Nuheara’s newly developed hearing aid products, currently undergoing clinical trials, to be offered to US customers under the recent FDA changes to OTC hearing aid regulations.
Commenting on Nuheara’s expanded relationship with Best Buy, Mr Miller said:
“Our relationship with Best Buy in North America across the last five years has enabled Nuheara to deeply understand key consumer insights in relation to hearing health. With our traditional retail sales rapidly growing, this in store expansion is a further example of the strong momentum we are seeing return to this segment of the retail channel.”
“We are very excited to be working closely with Best Buy as it offers hearing solutions online and in select US-based Best Buy retail stores. It is important for Nuheara’s customers that our hearing products can now be researched and purchased at Best Buy online for home delivery, hybrid via click and collect, and directly from one of these brick-and-mortar stores with information displays,” added Mr Miller.
GLOBAL SUPPLY AGREEMENT WITH SONOVA
In December 2021, Nuheara announced that it had secured a three year Global Supply Agreement (Agreement) with Switzerland-based company Sonova AG (Sonova). Under the Agreement, the Company will supply Nuheara manufactured and branded products to Sonova and its affiliates worldwide.
Headquartered in Stäfa Switzerland, Sonova is the largest manufacturer of hearing aids in the world, and the second largest globally in audiological care. The group operates through its core hearing aid brands and its Audiological Care business brands – AudioNova, Geers, Boots Hearing Care, Lapperre, World of Hearing, and Connect Hearing. Sonova reaches its customers through multiple channels, including online and via clinics / Points of Sales (POS) in over 100 countries globally, including in Australia operating under the Connect Hearing brand.
Under the Agreement, Nuheara will initially supply its products to Sonova entities in the US and Australia. Other global regions will follow, with Europe, UK and Canada the priorities, as Nuheara establishes trading entities in those regions/countries to extend the deployment globally.
COMMENCEMENT OF MEDICAL DEVICE CLINICAL TRIAL AND BIOCOMPATIBILITY TESTING, PROVIDING PATHWAY TO 510(K) SUBMISSION
In October 2021, Nuheara announced that it had commenced a medical device clinical trial to test the safety and effectiveness of the Company’s newly developed range of hearing aid products, to support Nuheara’s planned expansion into clinically tested and regulatory approved medical devices.
Nuheara engaged National Acoustics Laboratories (NAL) under the terms of a Clinical Trial Agreement (CTA) to assist with conducting the clinical trial.
The results of the clinical trial will support Nuheara expanding its global hearing solutions by meeting hearing aid compliance requirements initially with the US FDA, the European Union (EU Mark) and the Australian Therapeutic Goods Administration (TGA).
On 25 November, a further announcement was made advising that recruitment of candidates for the trial had been completed and that 50% of the candidates had already commenced.
On successful completion of the trial, Nuheara plans to proceed with a 510(k) submission to the FDA for approval of a Class II, self-fitting air conduction, wireless hearing aid – 21 CFR Part 874-3325, Product Code: QDD (Self-fit Category). This FDA submission is expected to be made during Q3 FY22.
Running concurrently with the clinical trial, biocompatibility testing and UL certification is also being completed. To conduct Cytotoxicity, Irritation and Sensitisation testing, Nuheara engaged NAMSA, the world’s only 100% medical device-focused contract research organisation, and a US FDA ASCA-accredited medical device biocompatibility laboratory. The testing was conducted in accordance with ISO standards, to demonstrate biocompatibility of Nuheara’s proposed hearing aid devices. In accordance with these standards, the following excellent results were recorded:
• Cytotoxicity testing was found to meet requirements
• Irritation testing outcomes resulted in a Primary Irritation Index of zero.
• Sensitisation testing showed no evidence of contact sensitisation.
The planned 510(k) submission also supports the Company’s plan to secure broad FDA self-fit hearing aid clearance in time to meet the expected demand for a new category of the OTC hearing aids in the US, which specifically target individuals with mild-to moderate hearing loss.
NUHEARA UNDERTAKES CAPITAL RAISING TO SUPPORT GROWTH
Following a series of achievements over 2021 that demonstrated ongoing market progress and increased sales, Nuheara undertook a successful capital raising to fund immediate growth opportunities, particularly in the US, comprising:
• $3.0 million invested by a United States-based healthcare related fund;
• $1.6 million private placement from existing and new professional sophisticated investors; and
• $1.1 million Share Purchase Plan that provided the opportunity for the Company’s shareholders to further invest on the same terms as the private placement.
Having put in place key foundational relationships and a strong omni-channel network, Nuheara is well positioned to execute on opportunities in key growth markets, particularly the US, in calendar year 2022.
The US hearing market provides a substantial opportunity that warrants significant further focus and investment. This will be the key focus of Nuheara’s activity and expenditure over the next 12 months.
RECEIPT OF $1.74 MILLION R&D TAX CASH REBATE
On 1 December 2021, Nuheara announced the receipt of a $1.74 million Research and Development (R&D) Tax Incentive cash rebate from the Australian Tax Office. The rebate reflects Nuheara’s longstanding and ongoing commitment to be at the forefront of industry innovation and provide research leadership in the rapidly changing landscape of global hearing healthcare and leading-edge medical devices.
EXPENDITURE
Research and development
Research expenditure that is directly attributable to development activities is capitalised as an intangible asset under Australian Accounting Standards. As a result, expenditure of $1.3 million has been capitalised this quarter (Q2 FY22), representing 35% growth on the same quarter last year (Q2 FY21: $0.9 million) and is shown as “Payments to Acquire Intellectual Property” under cash flows from investing activities at item 2.1(e). This movement is mainly attributable to work on new generation of products, including work performed towards accreditation as a medical device Company.
Product manufacturing and operating costs
The sale of IQbuds2 MAX and accessory products continued during the quarter. Orders have been placed for ongoing production runs for the remainder of the calendar / financial? year. Cash outlay of $1.1 million in Q2 FY22, down 35% over the comparative quarter last year (Q2 FY22: $1.7 million), is predominantly attributable to payment for completed units.
Advertising and marketing
Advertising and marketing spend of $1.0 million in Q1 FY22 was on par over the same quarter last year (Q2 FY21: $1.0 million). Advertising and marketing spend is directed towards the generation of online sales (DTC) and traditional retail sales channel support.
Staff costs
Consistent with R&D expenditure noted above, employment expenses related to employees working on R&D activities are also capitalised as an intangible asset under Australian Accounting Standards. Staff costs of $1,249k in Q2 FY22 represented a 100% increase over the same quarter last year (Q2 FY21: $625k) with movement mainly attributable to the timing of payments and amounts capitalised.
The remaining staff costs represent corporate, operations, finance, administration, and marketing employees, including related party payments for non-executive Director fees, and salaries paid to executive Directors during the period (refer item 6.1).
Payments to related parties in Q2 FY22 were $208k, which related to fees paid to non-executive directors and the executive directors’ cost of payroll for the period.
Administration and corporate costs
Administration and corporate costs of $0.6 million in Q2 FY22, were 45% lower than the same quarter last year (Q2 FY21: $1.1 million). The movement relates mainly to the timing of creditor payments.
MINERAL ASSETS
There has been no change in mineral assets held during the quarter.
Nuheara’s remaining mining asset consists of an 80% interest in a Net Smelter Royalty located in Northern Peru, held by its subsidiary Terrace Gold Pty Ltd. Nuheara intends to divest the asset as soon as it is commercially practical to do so.
INVESTOR BRIEFING DETAILS
Justin Miller (Co-founder & CEO) and Jean-Marie Rudd (CFO) will host an investor webinar at 12:30pm AEDT / 9.30am WST on Tuesday 1 February 2022. Following the presentation, participants will have an opportunity to ask them questions.
To attend the webinar, please pre-register at:
https://us02web.zoom.us/webinar/register/WN_ylvqRI30QTKqq58D007w5w
AUTHORISED BY:
Justin Miller
Managing Director and CEO Nuheara Limited
MEDIA – US:
Maura Yepez, Firebrand
Email: [email protected] Phone: +1 415 848 9175
INVESTORS:
Ronn Bechler, Market Eye
Email: [email protected] Phone: + 61 400 009 774
MEDIA – AUSTRALIA:
Ranya Alkadamani
Email: [email protected] Phone: +61 434 664 589
ABOUT NUHEARA
Nuheara is a global leader in smart hearing technology which change people’s lives by enhancing the power to hear. As a global pioneer in Hearable products, Nuheara has developed proprietary, multi-functional, personalised intelligent hearing devices that augments a person’s hearing. Nuheara is headquartered in Perth, Australia and was the first consumer wearables technology company to be listed on the Australian Stock Exchange (ASX).
In 2016, the Company released its revolutionary wireless earbuds, IQbuds, which allow consumers to augment their hearing according to their personal hearing preferences and connect hands free with their voice-enabled smart devices. In 2020 Nuheara released its third generation IQbuds² MAX. In 2021, Nuheara transformed its operations to include medical device manufacturing for its hearing aid products to meet global demand for mild to moderate hearing loss. Nuheara products are now sold Direct to Consumer (DTC) and in major consumer electronics retailers, professional hearing clinics, pharmacies and speciality retailers around the world. Nuheara products are now sold Direct to Consumer (DTC) and in major consumer electronics retailers, professional hearing clinics, pharmacies and optical chains around the world.
The Company’s mission is to transform the way people hear by creating smart hearing solutions that are both accessible and affordable.
For further information, please visit https://www.nuheara.com/.


