- Nuheara welcomes De Novo status for a self-fitting hearing device
- Nuheara continues its strategy of unlocking affordable and accessible hearing solutions
- Globally renowned Hörzentrum Oldenburg GmbH commissioned to complete study on IQBuds Boost™ impact on improving consumers’ hearing
17 October 2018 – Perth, Australia
Nuheara Limited (ASX: NUH) (“Company” or “Nuheara”), transforming the way people hear by creating game-changing hearing solutions that are accessible and affordable, welcomes the recent move the US Federal Drugs Administration (FDA) in approving De Novo status for a self-fitting hearing device.
The De Novo process provides an alternative pathway for medical device manufacturers to classify novel medical devices that can be shown to be safe and effective for consumer use and which does not currently “fit into” any of the current FDA Class I or Class II medical device categories.
As a result of the De Novo approval secured by Bose Corporation (Bose) of the Bose Hearing Aid, Nuheara will also assess this alternative regulatory pathway to potentially market a self-fitting hearing aid. The Company will do this once the FDA has released both the Special Controls document, which governs the requirements applicable to subsequent companies, as well as the details of the Bose clinical study. Both documents are expected to be released by the FDA within the next month.
While the De Novo approved hearing aid has not been launched nor shown to market, this action by a major electronics manufacturer – outside of the six major hearing aid manufacturers that control 95% of the world’s hearing aid market – has brought valuable focus for the growing hearing healthcare channel. Nuheara has been driving the expansion of this growing channel by developing accessible and affordable smart hearing solutions to an underserviced mild-to-moderate hearing loss market.
Bose is yet to release any information on the product’s form factor, functionality, price, and availability. Importantly, the Bose Hearing Aid is not classified as an Over-The-Counter (OTC) hearing aid and subsequently must still follow the same regulations as all other hearing aids currently on the market.
OTC regulations are still being formulated by the FDA and are due for release in August 2020. Once the regulations are released and understood, Nuheara will take a strategic stocktake of its product line-up and determine if its products need to be tailored or further developed to ensure they fit within the OTC regulations.
Further, in September 2018, in a proactive move to validate the broader hearing market potential of IQbuds BOOST™, Nuheara commissioned the internationally acclaimed Hörzentrum Oldenburg GmbH to conduct independent research on the consumer usage of IQbuds BOOST ™ and its impact on improving consumers’ ability to hear better. As the first step towards a definitive clinical study, this validation is expected to be completed in November 2018.
“Hörzentrum is a globally renowned audiological research and development organisation, known or working with the leading hearing aid companies on hearing technological and consumer research,” said Justin Miller, CEO of Nuheara.
“Nuheara will continue to develop and market affordable and accessible assistive hearing devices, for the hearing healthcare market, that are categorised as Smart Hearing products. While we have not seen the form factor nor pricing for Bose’s entry into the hearing aid market, we are delighted that a global reputable company beyond the traditional hearing aid manufacturers will be a player in the evolving $8 billion market.”
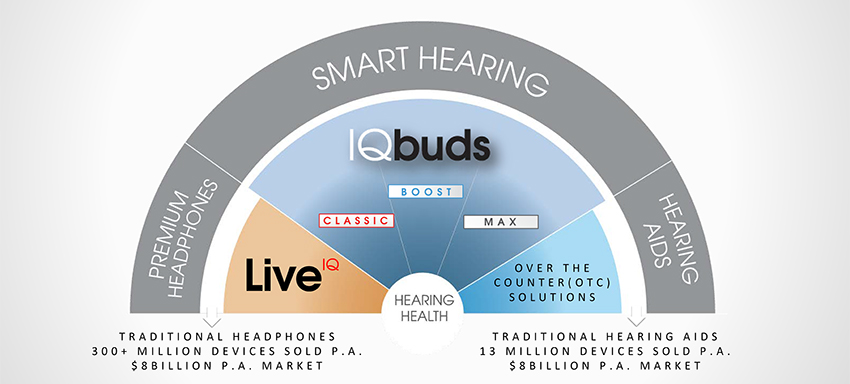
Figure 1: Nuheara’s Smart Hearing target market in relation to headphone and hearing aid markets.
CONTACTS
Australia
Justin Miller
CEO and Managing Director
+61 8 6555 9999
[email protected]
Media
Ranya Alkadamani
+61 434 664 589
[email protected]
About Nuheara
Nuheara is a global leader in Intelligent Hearing: smart personal hearing devices that enhance and amplify human experiences. Nuheara has developed proprietary and multi-functional intelligent hearing technology that augments a person’s hearing and facilitates cable free connection to smart devices. Nuheara is based in Perth, Australia and has offices in San Francisco and New York, USA. Nuheara was the first consumer wearables technology company to be listed on the Australian Stock Exchange (ASX).
In 2016, the Company released its revolutionary wireless earbuds, IQbuds™, which allow consumers to augment their hearing according to their personal hearing preferences and connect hands free with their voice-enabled smart devices. IQbuds™ are now sold in major consumer electronics retailers and professional hearing clinics around the world. The Company’s mission is to improve people’s lives by allowing them to seamlessly listen, communicate, and connect to their physical and digital worlds.
Learn more about Nuheara: www.nuheara.com


